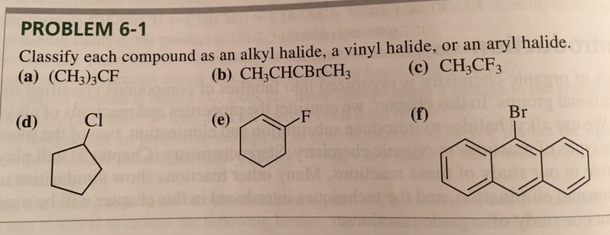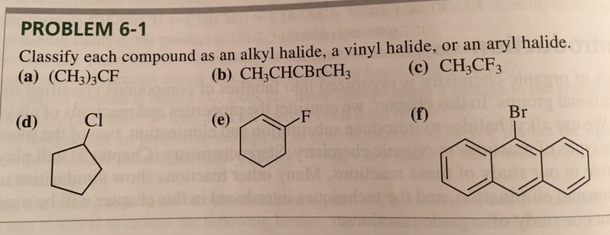Start with an alkyl halide and.
What is a vinyl alkyl halide.
Methyl iodide and ethyl chloride.
The functional group of alkyl halides is a carbon halogen bond the common halogens being fluorine chlorine bromine and iodine.
Halogens are treated the same way as alkyl groups.
For example if the halogen atom is attached to a carbon atom which is attached to a benzene ring cl ch 2 c 6 h 5 one would think it is an aryl halide but it is an alkyl halide because the halogen atom is attached to the carbon that is sp 3 hybridized.
In aryl halides the halogen bearing carbon is part of.
In vinylic halides the carbon that bears the halogen is doubly bonded to another carbon.
Other articles where tertiary alkyl halide is discussed.
There is an interaction between one of the lone pairs on the chlorine atom and the delocalized ring electrons and this strengthens the bond.
They are subdivided into alkyl vinylic aryl and acyl halides.
Alkyl halides fall into different classes depending on how the ha.
Halogens are more electronegative than carbon.
The name of the halogen is followed by the name of the.
In the generally accepted nomenclature of alkyl halides the name of the alkyl residue is followed by the name of the halide e g.
With the exception of iodine these halogens have electronegativities significantly greater than carbon.
An aryl halide has general formula c 6h 5x in which an halide group x has substituted the aryl ring.
In a primary alkyl halide the carbon that bears the halogen is directly bonded to one other carbon in a secondary alkyl halide to two and in a.
The iupac nomenclature shown in brackets in the illustration below considers an alkyl halide a substituted alkane i e.
The carbon chlorine bond in chlorobenzene is stronger than you might expect.
Finally halo is prefixed to it.
Classified as primary secondary or tertiary according to the degree of substitution at the carbon to which the halogen is attached.
Structure and physical properties.
Vinyl or more specifically pvc is also used in plastic pipes synthetic leather records hence the name vinyl record insulation and many other products.
A vinyl halide is clearly a species with a formula h 2c c x h in which a halide is directly bound to an olefinic bond formally this is ethylene h 2c ch 2 with one of the hydrogens substituted by a heteroatom vinyl chloride h 2c chcl is an example.
Iupac nomenclature of alkyl halide or haloalkanes in substitutive system of iupac nomenclature the alkyl halides are named as haloalkanes.
In alkyl halides all four bonds to the carbon that bears the halogen are single bonds.
In this system a root word is chosen based on the number of carbon atoms present in the parent chain and then the primary suffix ane is added.
However alkyl halides may sometimes be confused with aryl halides.
The extra strength of the carbon halogen bond in aryl halides.